What’s the best way to make a Mars Microbe?
How ‘should’ you do bacterial directed evolution?
Evolution has succeeded at thriving in nearly every corner of Earth, from sub-zero brines to toxic deserts. That’s a great precedent for our mission to develop life beyond our planet. The problem is that it might happen far too slowly for a human timeline. Left to its own devices, evolution would need millions of years and oceans of organisms to adapt to another planet. To grow life on Mars, we need results in months, not millennia.
To accelerate evolution to the human timescale, we developed new techniques to access new kinds of genetic diversity that we hoped would perform better than natural mutation. The issue with evolution in the wild is that genetic diversity arises over time from natural mutation, which is slow, limited to the DNA already present in the organism, and gives an unfair advantage to mutations that happen to occur first. We designed new techniques to address these issues, aiming to achieve better outcomes than Adaptive Evolution.
To our surprise, plain old Adaptive Evolution gave us the best results after a single cycle, picking the “low-hanging fruit” more effectively than our new engineering strategies. This suggests that we should let cells self-optimize first, achieving easy wins before applying our more advanced techniques.
🐎🏆 We did a side-by-side competition of three evolution techniques
Our three techniques are:
Adaptive Evolution, where diversity slowly arises from natural mutation. This most closely mimics the process in the wild and is the only technique where you diversify and select at the same time. It tends to produce multiple mutations per cell, not all of which actually matter.
BioBloom is a technique that aims to perform barcoded, saturating mutagenesis genome-wide: creating every possible single mutation first, then performing selection afterward. This tests more mutations faster than Adaptive Evolution and produces richer data about which ones work. But each cell only gets one mutation.
Additive Engineering instead adds DNA rather than changing it. We add a library of DNA from other organisms, giving our organism access to new genes or pathways it didn’t have before. Instead of evolving existing genes one mutation at a time, this method gives you access to completely new genes.
To test how best to evolve microbes for Mars, we ran a competition between these three evolution techniques to benchmark them against each other. We ran each method through a single cycle1 focused on improving E. coli’s salt tolerance, which is a major component of Mars stress. The goal wasn’t to create a winning bacterial strain. The goal was to quantify what each approach delivers on a realistic timescale and to look under the hood at how and why those gains are achieved. Here’s what we found:
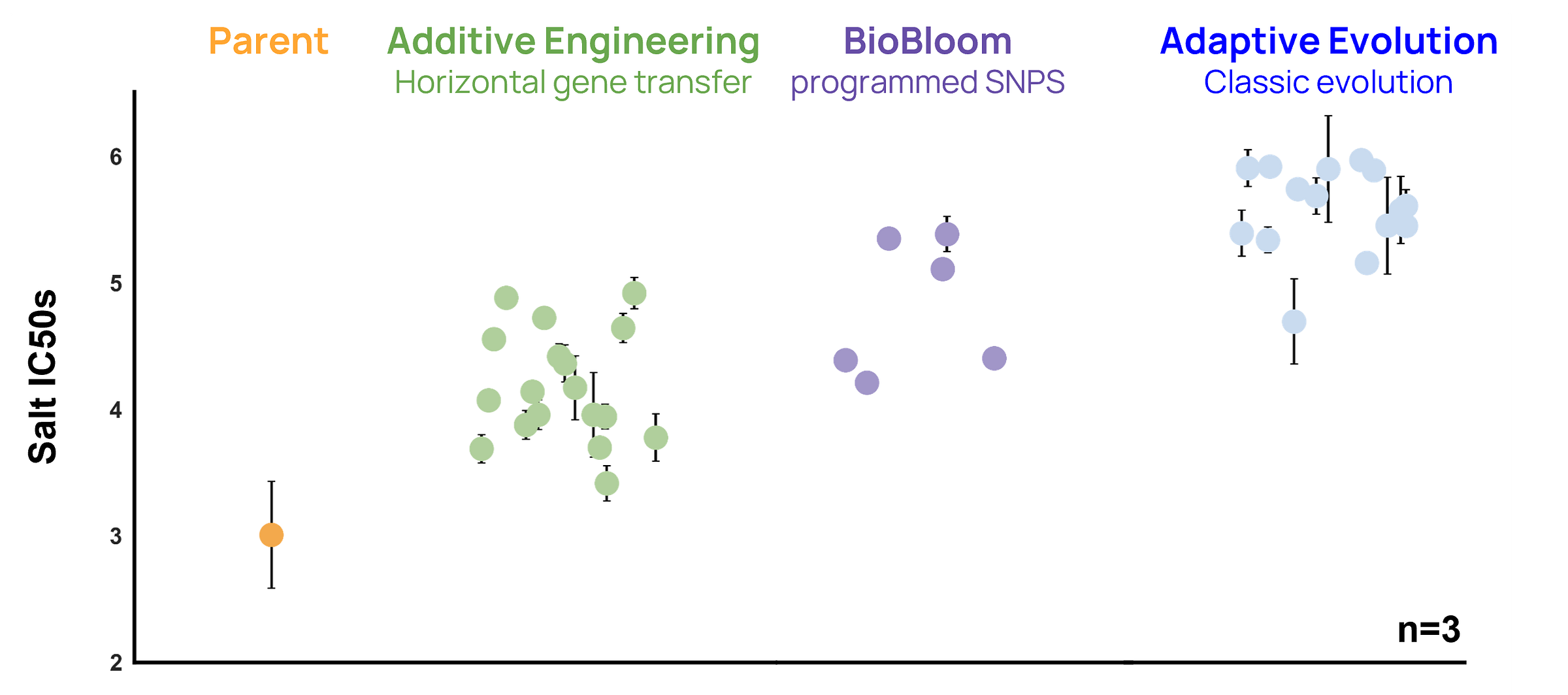
Salt tolerance measured with an IC50 assay. Each of the three evolution techniques generated new strains that exhibit higher salt tolerance than the original wildtype “Parent” strain.
The headline result is simple and a little surprising: across this window, all three methods delivered similar absolute improvements, within about 1.5x of each other in terms of ∆IC50. In the figure above, you can see Adaptive Evolution sitting at the top by a modest margin, BioBloom close behind, and Additive Engineering improved but behind the other two on the first pass. This tells us that all three techniques are effective, and we should consider all of them as we make a Mars Microbe. The question of which one is ‘best’ is more nuanced, as they pull different levers, in different ways, with big differences in laboratory implementation. Let’s dive in!
🍎🍊Comparing these techniques is nuanced because they’re apples-and-oranges
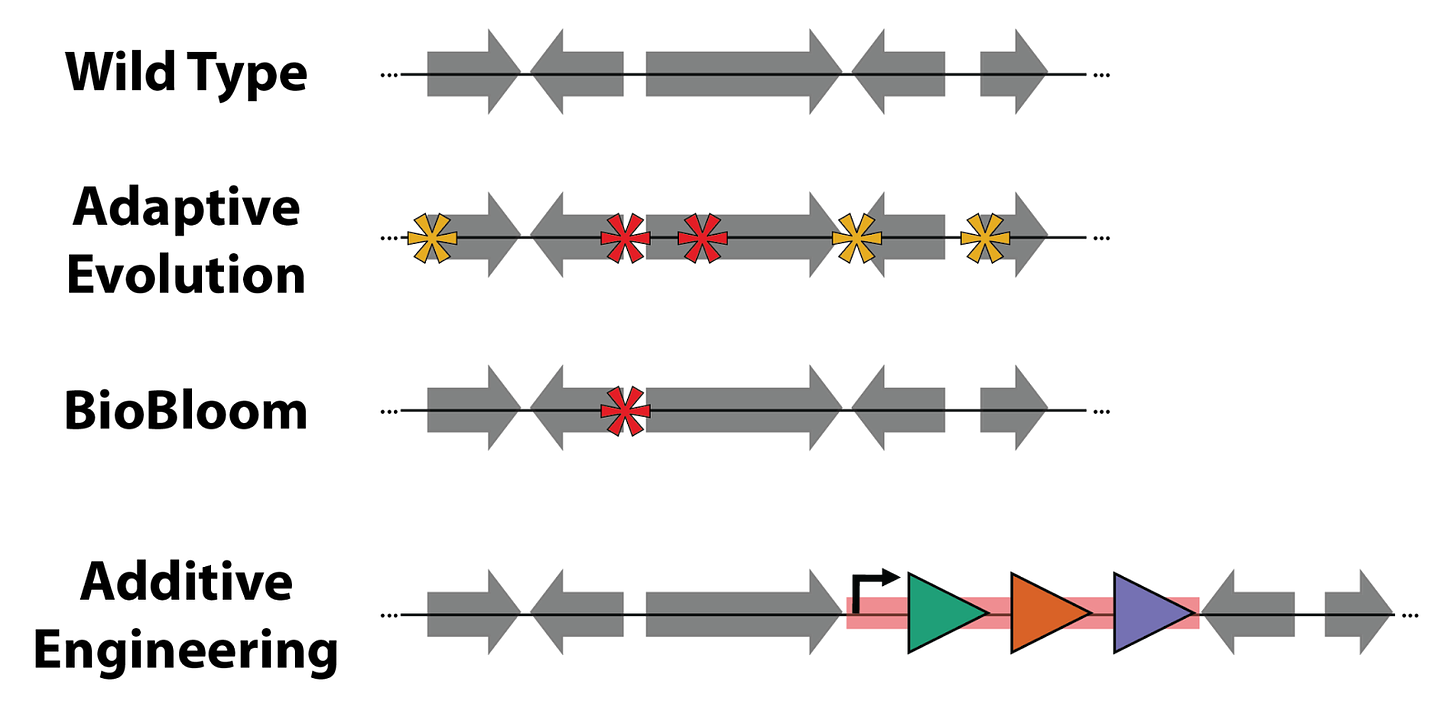
Each method results in strains with different types of genetic variation. Taking a peek under the hood, Adaptive Evolution will result in multiple single-nucleotide polymorphism (SNP) mutations, only some of which matter. BioBloom will make a single SNP that’s much more likely to be optimal. Additive Engineering will add new, horizontally transferred DNA that confers some benefit2.
The evolution techniques differ in the kinds of genetic variation they introduce.
The methods are also asymmetric in terms of difficulty, pace, and the type of human effort required. Adaptive Evolution requires a short amount of hands-on time every day to ‘passage’ bacteria, that is, initiate growth of a new bacterial culture with a little bit of yesterday’s bacteria. Fundamentally, Adaptive Evolution is limited by time, as bacteria divide only so quickly3. In contrast, BioBloom and Additive Engineering both require more sophisticated molecular biology upfront, but then can repeat cycles of diversification and selection faster thereafter4.
To discuss the outcomes of each experiment in a little bit more detail:
Adaptive Evolution gave us the largest bump in salt tolerance simply by letting E. coli grind through a month of daily passaging - including weekends! Random SNPs piled up: some helpful, some hitchhiking, and the net result outperformed the other approaches, but at the price of time and genetic clutter. Because every winning clone carries several changes, we still have to disentangle which alleles actually matter. Some of them are likely to be neutral or even negative hitchhiker mutations, which can make strains brittle and overspecialized to the selection environment. We also expect the benefits of Adaptive Evolution to plateau over longer time periods; diminishing returns are predicted by evolutionary theory and have been seen in other projects.
BioBloom doesn’t wait for mutations. We built a barcoded library containing virtually every possible point mutation, ran the whole set through selection in a few days, and read out the tags to spot the strongest single-site winners. Those clones are less fit than the multi-mutant strains from Adaptive Evolution, but they reveal a broader set of important genes than we saw in Adaptive Evolution, and can quantify the actual fitness of each mutation. The catch is that BioBloom currently only works in E. coli and requires substantial upfront effort before it can be used in a new organism.
Additive Engineering tries a different angle - dropping in foreign DNA rather than tweaking what’s there. We introduced libraries of extremophile DNA into cells via plasmids or transposons and observed modest gains for both approaches. But most of the transposon-integration winning strains turned out to have random pieces of DNA that inserted into the very same loci already flagged by Adaptive Evolution and BioBloom. True “new pathway” wins were present, but not in the majority. They’ll probably shine later, once native mutations hit their ceiling. But in this experiment, horizontal gene transfer proved less valuable than simple SNPs.
In summary, it’s tough to come up with a completely fair way to compare these three techniques because they’re apples-and-oranges in terms of speed, amount of effort, and the nuance of how widely each method can be applied in diverse organisms5. But doing a side-by-side bakeoff gives us a benchmark for comparison and deeper insight into how each method works, along with lessons about how to apply them.
🔎🗺️ Takeaways on how to evolve Mars microbes
Our goal is to accelerate evolution to adapt life to a new planet on a human timescale, not a geologic one. From the above experiments, we’ve learned that you should start with Adaptive Evolution because it’s quick to launch, easy to scale, and reliably harvests the “low-hanging” mutations that give the biggest early pay-offs. Any strain that comes out of this phase may need some cleanup and validation, potentially with BioBloom to help isolate which SNPs matter and which are hitchhikers. Then, with the native genome polished, we can use Additive Engineering to introduce new pathways and surpass the limits of the original organism.
Past that, we’re back in speculative territory. The best overall strategy is probably to switch between Additive Engineering and Adaptive Evolution, integrating a batch of novel genes before running a short cycle of Adaptive Evolution to domesticate them. Inserts that merely tweak existing regulation will fade, leaving pathways that genuinely extend the toolkit to help a Mars microbe thrive under poly-extreme stress.
💽💻Could AI do even better? Stay tuned!
All of the work above explores how well different experimental techniques can generate improved strains. One major missing piece is iteration. Each time you evolve an organism, you gain data that can help you next time. Closed-loop experimentation can take many forms, but we’re especially interested in whether AI methods can utilize the vast datasets generated by BioBloom and Additive Engineering to speed up the process.
For BioBloom, we’re writing a more detailed technical report that will be coming out soon. There, we’ll elaborate on the technique, characterize the coverage and statistical power of the library, and dive into some of the specifics of our results for this salt selection. Stay tuned and subscribe to our substack to get notified when this technical report is released!
For Additive Engineering, we’re currently working on a proposal for a large-scale dataset that will create a “microbial phenomics atlas” by characterizing the fitness of thousands of genes from hundreds of organisms across multiple hosts and conditions. Based on these results, we’re planning to do that screening with plasmids. We’re still seeking technical commentary on the proposal, so please reach out if you would like to get involved.
And as always, we’d love to discuss these results and their interpretation. Drop us a comment below!
For Adaptive Evolution, a “cycle” is one month of continuous passaging. For the other two techniques, it’s a single round of mutagenesis and selection.
The DNA for Additive Engineering is randomly fragmented genomic DNA from eleven different extremophiles (variously halophiles, radiophiles & cryophiles) constructed as previously discussed. The insert size ranges from 1.5 to 8 kb, with a median of 2.5 kb. Our libraries are over 10^6 in size, providing roughly 50x coverage of all 11 donor genomes.
We built and tested two versions of this library: one in which we integrated the new DNA randomly into the genome using a transposon, and another in which we expressed it from a plasmid.
This experiment lasted 30 passage cycles, with a daily culture volume of 1 mL and 50 uL transfer volumes, for a total of ~130 generations. We did not use additional mutagenesis. We could increase culture and transfer volumes to achieve more rapid evolution in the future.
For BioBloom, it takes 1 day to transform the libraries into cells, followed by 2 days of genomic editing and 2 days of selection in salt. For Additive Engineering, it takes 1 day to conjugate the library in and then 3 days of selection in salt.
Adaptive Evolution can be applied to any organism you can culture. BioBloom is the most restrictive, requiring plasmids, high-efficiency transformation, and the ability to use recombineering tools. This is why it’s currently only functional in E. coli. Additive Engineering is performed by conjugation, using a method similar to transposon knockout screening. It can easily be extended to most gram-negative bacteria without cloning new libraries, and we currently have it functioning in four diverse organisms of interest.



Interesting that old school serial dilution ALE performs the best. How were you changing the selection pressure - a linear increase in salt concentration each day or something else?
Nobody dares to read this! Are you one of them?
https://thegonersclub.substack.com/p/consciousness-is-a-trick-of-meat